Swedish nuclear medicine software developer Hermes Medical Solutions has received U.S. Food and Drug Administration (FDA) clearance for its latest Hybrid Viewer 7.0 software.
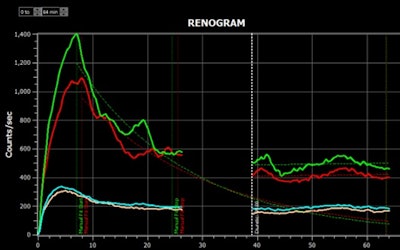
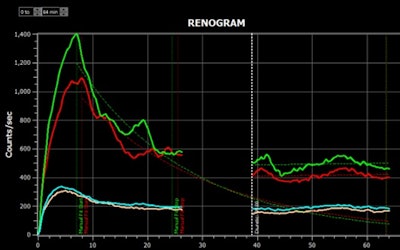 Results of a renogram displayed by the Hybrid Viewer 7.0 software.Image courtesy of Hermes Medical.
Results of a renogram displayed by the Hybrid Viewer 7.0 software.Image courtesy of Hermes Medical.
The software is for reconstructing and processing nuclear medicine imaging studies. It simplifies workflows significantly and supports all camera brands, the vendor said. The software is also cleared in Europe and Canada. The latest updates streamline workflows and strengthen compatibility with the camera models from all vendors and include the following:
Whether you are a professional looking for a new job or a representative of an organization who needs workforce solutions - we are here to help.