The 2024 version of the LI-RADS Ultrasound Surveillance system improves detection of hepatocellular carcinoma (HCC) over earlier iterations, a study published January 22 in the American Journal of Roentgenology found.
Researchers led by Sang Hyun Choi, MD, PhD, from the University of Ulsan in Seoul, South Korea found that LI-RADS version 2024 (v2024) leads to higher sensitivity but lower specificity for HCC detection compared to LI-RADS version 2017 (v2017). They noted that the increased sensitivity relates to increased alpha fetoprotein (AFP).
“These findings support transition from v2017 to v2024 to improve HCC detection in at-risk patients undergoing surveillance,” Choi and co-authors wrote.
The American College of Radiology (ACR) updated its LI-RADS Ultrasound Surveillance algorithm in 2024. The latest version incorporates AFP data and a visualization score named VIS-C into management recommendations after nonpositive results.
The Choi team compared the diagnostic performance of LI-RADS v2024 with that of LI-RADS v2017 for detecting HCC in at-risk patients. It also identified predictors of VIS-C scoring on follow-up surveillance exams.
The retrospective study included data collected from a prospective trial that took place in 2011 and 2012. Final analysis included 407 patients with a median age of 56 years. Of these, 230 were male and 177 were female. The patients had cirrhosis and underwent semiannual surveillance ultrasound.
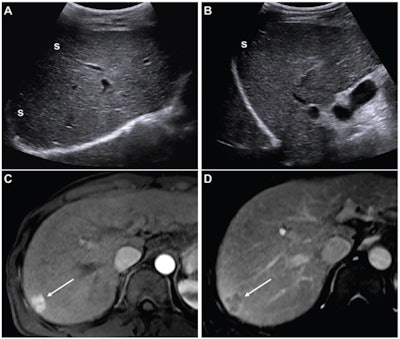
 (A,B) Images from an initial ultrasound examination showed no identifiable lesions and received a consensus ultrasound category of US-1. Nearly the entire liver is visualized, with minimal shadowing (“s”, A and B), and the examination received consensus result of VIS-A. The AFP level was 7.1 ng/mL on assessment six months before the ultrasound, 9.1 ng/mL on assessment three months before examination, and 14.2 ng/mL on assessment on exam day. The AFP result was thus assessed as positive based on a progressive increase on two consecutive tests. The atient was considered to have negative surveillance result according to LI-RADS Ultrasound Surveillance v2017, but positive surveillance result according to LI-RADS Ultrasound Surveillance v2024. (C-D) Axial arterial-phase (C) and portal-venous phase (D) T1-weighted images from subsequent gadoxetic acid-enhanced MRI show a 2-cm arterial-phase hyperenhancing observation (arrow, C) with portal-venous washout (arrow, D) in segment 7. This observation was classified on MRI as LR-5, thus meeting the present study’s reference standard for diagnosis of HCC. The mass is located in the periphery of the liver, an anatomic area where ultrasound visualization can be challenging due to shadowing.ARRS
(A,B) Images from an initial ultrasound examination showed no identifiable lesions and received a consensus ultrasound category of US-1. Nearly the entire liver is visualized, with minimal shadowing (“s”, A and B), and the examination received consensus result of VIS-A. The AFP level was 7.1 ng/mL on assessment six months before the ultrasound, 9.1 ng/mL on assessment three months before examination, and 14.2 ng/mL on assessment on exam day. The AFP result was thus assessed as positive based on a progressive increase on two consecutive tests. The atient was considered to have negative surveillance result according to LI-RADS Ultrasound Surveillance v2017, but positive surveillance result according to LI-RADS Ultrasound Surveillance v2024. (C-D) Axial arterial-phase (C) and portal-venous phase (D) T1-weighted images from subsequent gadoxetic acid-enhanced MRI show a 2-cm arterial-phase hyperenhancing observation (arrow, C) with portal-venous washout (arrow, D) in segment 7. This observation was classified on MRI as LR-5, thus meeting the present study’s reference standard for diagnosis of HCC. The mass is located in the periphery of the liver, an anatomic area where ultrasound visualization can be challenging due to shadowing.ARRS
Two radiologists independently assigned ultrasound categories to exams performed in the first round of surveillance and visualization scores to exams performed in the first and second rounds. A third radiologist meanwhile settled disagreements between the first two radiologists.
The team considered AFP to be positive if it was elevated or increasing from pre-enrollment values. It used positive biopsy or LR-5 observations on MRI as reference standards.
Of the total patients, 28 had an HCC diagnosis. The two radiologists showed higher sensitivity and lower sensitivity when using LI-RADS v2024 compared with v2017.
| Diagnostic performance of radiologists using LI-RADS | ||
|---|---|---|
| Measure | Radiologist 1 | Radiologist 2 |
| LI-RADS v2024 | ||
| Sensitivity | 64.3% | 64.3% |
| Specificity | 82% | 82.3% |
| LI-RADS v2017 | ||
| Sensitivity | 42.9% | 39.3% |
| Specificity | 92.6% | 92.9% |
| *All data achieved statistical significance. | ||
Seven patients had their HCC detected by LI-RADS v2024, but not v2017 when using consensus assessments. These patients had an increasing AFP while two also had elevated AFP.
Of the total study cohort, 299 patients underwent a second round of ultrasound exams after a negative first round using v2024. Here, the only independent predictor of the second round VIS-C was first round VIS-C, the team reported. This included an adjusted odds ratio of 21 (p
Finally, in 88 patients with first round VIS-C, no LI-RADS v2024 risk factor showed significant univariable association with repeat VIS-C scoring.
The study authors suggested that for the best use of LI-RADS v2024 in clinical settings, AFP levels should be routinely checked in patients. They added that radiologists performing and interpreting liver ultrasound exams should incorporate AFP results into their reports.
“This approach will help achieve a more integrated surveillance framework by bridging imaging and biochemical markers, facilitating multidisciplinary care,” they wrote.
The full study can be accessed here.
Whether you are a professional looking for a new job or a representative of an organization who needs workforce solutions - we are here to help.