CHICAGO – Siemens Healthineers shined the spotlight on its new expanded photon-counting CT portfolio and the latest version of its Acuson Sequoia ultrasound scanner in its booth at RSNA 2024.
CT
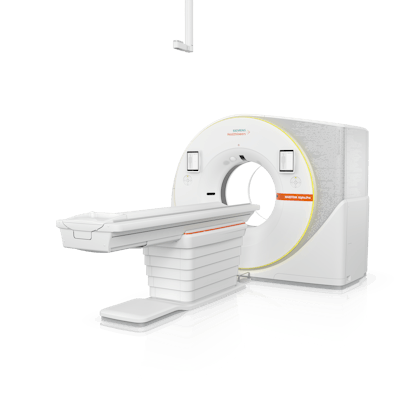
Siemens debuted a new dual-source scanner and a single-source system as part of its new Naeotom Alpha class of photon-counting (PCCT) offerings.
Naeotom Alpha.Pro is a dual-source scanner, while Alpha.Prime will be the vendor’s first single-source PCCT system. Meanwhile, the company’s flagship Naeotom Alpha scanner has been refreshed with new hardware and software and has been renamed as Alpha.Peak.
 Naeotom Alpha is Siemens' new dual-source PCCT scanner. Image courtesy of Siemens Healthineers.
Naeotom Alpha is Siemens' new dual-source PCCT scanner. Image courtesy of Siemens Healthineers.
All three Alpha scanners feature the company’s spectral imaging capabilities and AI and automation features. They also all utilize Siemens’ Quantum HD technology, which can display anatomical details in high resolution at a slice thickness of 0.2 mm, according to the vendor.
Ultrasound
Siemens also highlighted the latest version of its Acuson Sequoia ultrasound system featuring new hardware and AI-powered capabilities designed to improve workflows and efficiency.
Launched in December 2024, the Acuson Sequoia 3.5 boasts AI Abdomen, which automatically labels and measures organs in milliseconds and reduces sonographer hand motion by up to 89% compared with manual exams and up to 44% compared with protocol exams, the company said. This improved ergonomics can benefit up to 90% of ultrasound users who have reported scanning while in pain related to pressure applied to the transducer, abduction of the arm, and twisting of the neck and trunk, Siemens said.
The AI Abdomen software automatically recognizes and labels 17 anatomical views and calculates 12 key measurements, which may improve both exam and reading workflows and protocol standardization, the company noted. Acuson Sequoia 3.5 has proprietary 2D Shear Wave technology that can identify and display stiff lesions in the breast, while its new HLX transducer provides penetration and resolution, which can help clinicians reduce false negatives, Siemens said.
 Acuson Sequoia 3.5 features new hardware and AI capabilities. Image courtesy of Siemens Healthineers.
Acuson Sequoia 3.5 features new hardware and AI capabilities. Image courtesy of Siemens Healthineers.
The new HLX transducer also addresses needs in musculoskeletal imaging, such as the need to visualize large in-motion tissues and small, superficial structures for conditions such as tendon tears and inflammatory arthritis. For liver imaging, Acuson Sequoia 3.5 includes automation tools for liver quantification techniques such as ultrasound-derived fat fraction and point shear wave elastography in a single acquisition, Siemens said.
In other news, Siemens noted that it has entered an agreement with RadNet subsidiary DeepHealth to embed DeepHealth’s SmartSonography software in the Acuson Sequoia line to further tackle workflow and workforce challenges for enhanced operational efficiency.
Molecular Imaging
The company’s Biograph Trinion PET/CT scanner and Symbia Pro.specta SPECT/CT scanner held prime space on the display floor at this year’s RSNA. The Biograph Trinion, first launched in June at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Toronto, Ontario, is a compact, scalable PET/CT scanner. The scanner was cleared for use in Europe at the end of September and orders for the system are picking up pace, the company said.
Meanwhile, the Symbia Pro.specta SPECT/CT scanner, cleared in Europe and the U.S. in June 2022, has been enabled with three new software packages specifically for applications in theranostics. The software provides options for optimizing treatment responses imaging protocols in patients with prostate cancer undergoing treatment with lutetium-177 (Lu-177) prostate-specific membrane antigen (PSMA)-617 and potentially other theranostic radiopharmaceuticals, Siemens said.
The packages come on newly ordered systems or can be installed on existing systems, Siemens said.
The vendor also showcased Biograph One, a work-in-progress PET/MR scanner. Biograph One, which has not yet received U.S. Food and Drug Administration (FDA) clearance, is designed to enable simultaneous visualization of organ location function in the body, function, and cellular metabolism – a capability that could open new possibilities in the field of theranostics.
MRI
Siemens presented a 70-cm bore version of its 1.5-tesla Magnetom Flow MRI scanner as a work in progress. With a focus on sustainability and AI, the scanner is expected to come with a flexible XL coil for larger body regions and require only 0.7 liters of helium. Siemens also plans to include its DeepResolve AI-based image reconstruction algorithms.
Both the 70-cm and 60-bore versions have not yet received FDA clearance.
Radiography/Fluoroscopy
In other work-in-progress demonstrations, Siemens introduced Luminos Q.namix, a next-generation radiography/fluoroscopy system with a focus on versatility and ergonomics. Two versions are expected to be produced: a remotely-controlled Q.namix R and Luminos Q.namix T with tableside control. Both systems have yet to receive FDA clearance.
Whether you are a professional looking for a new job or a representative of an organization who needs workforce solutions - we are here to help.