A new copper-61 (Cu-61)-labeled PET imaging agent shows promise for detecting disease in men with metastatic prostate cancer, according to a study published December 9 in Radiology.
The finding is from a phase I trial of Cu-61-NODAGA prostate-specific membrane antigen (PSMA) imaging and therapy (Cu-61 PSMA I&T) in eight patients, with the tracer yielding no adverse events and detecting more lesions than F-18 piflufolastat, noted lead author Gary Ulaner, MD, PhD, of the Hoag Family Cancer Institute in Irvine, CA, and colleagues.
“These data support the further development of Cu-61 PSMA I&T as a novel PSMA-targeting imaging agent,” the group wrote.
PSMA-targeted PET has revolutionized prostate cancer imaging, from initial staging of patients to identifying appropriate candidates for PSMA-targeted therapy, the authors wrote. To date, the U.S. Food and Drug Administration (FDA) has approved three tracers: gallium-68 PSMA-11, F-18 piflufolastat (Pylarify, Lantheus), and F-18 flotufolastat (Posluma, Blue Earth Diagnostics).
However, the short half-lives of the radioisotopes Ga-68 (68 minutes) and F-18 (110 minutes) limit the geographic range for their distribution, the authors noted. Conversely, Cu-61 has a half-life of 3.3 hours, which offers improved tracer biodistribution and greater image resolution, according to the researchers.
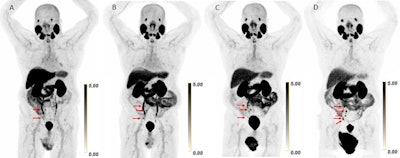 Scans in a 73-year-old man with biochemically recurrent prostate cancer (prostate-specific antigen level of 0.5 ng/mL) who underwent PET/CT with 9.4 mCi of F-18 piflufolastat and a second PET/CT examination with 8.1 mCi of copper 61-NODAGA (1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid)–prostate-specific membrane antigen for imaging and therapy (Cu-61 PSMA I&T) 24 days apart, with no intervening therapy. Maximum intensity projection (MIP) images obtained (A) 1 hour after F-18 piflufolastat administration and (B) 1, (C) 2, and (D) 4 hours after Cu-61 PSMA I&T administration are shown. Physiologic uptake is seen in the lacrimal glands, salivary glands, liver, spleen, kidneys, ureters, and bladder. Pathologic uptake in pelvic nodal metastases is labeled with arrows. The F-18 piflufolastat PET/CT MIP image reveals two avid pelvic nodal metastases (arrows). Cu-61 PSMA I&T PET/CT scans demonstrate three avid pelvic nodal metastases on 1-hour and 2-hour images (arrows); five avid pelvic nodal metastases were suspected on the 4-hour image (arrows).RSNA
Scans in a 73-year-old man with biochemically recurrent prostate cancer (prostate-specific antigen level of 0.5 ng/mL) who underwent PET/CT with 9.4 mCi of F-18 piflufolastat and a second PET/CT examination with 8.1 mCi of copper 61-NODAGA (1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid)–prostate-specific membrane antigen for imaging and therapy (Cu-61 PSMA I&T) 24 days apart, with no intervening therapy. Maximum intensity projection (MIP) images obtained (A) 1 hour after F-18 piflufolastat administration and (B) 1, (C) 2, and (D) 4 hours after Cu-61 PSMA I&T administration are shown. Physiologic uptake is seen in the lacrimal glands, salivary glands, liver, spleen, kidneys, ureters, and bladder. Pathologic uptake in pelvic nodal metastases is labeled with arrows. The F-18 piflufolastat PET/CT MIP image reveals two avid pelvic nodal metastases (arrows). Cu-61 PSMA I&T PET/CT scans demonstrate three avid pelvic nodal metastases on 1-hour and 2-hour images (arrows); five avid pelvic nodal metastases were suspected on the 4-hour image (arrows).RSNA
To validate its safety and performance in humans, the group conducted a trial between October 2024 and February 2025. It enrolled eight patients (mean age, 73 years old) with PSMA-avid disease first identified by F-18 piflufolastat PET/CT. Within 30 days, the participants also underwent Cu-61 PSMA I&T PET/CT at one, two, and four hours after injection.
Key results included the following:
Cu-61 PSMA I&T was well tolerated and demonstrated acceptable dosimetry.
The tracer enabled optimal imaging four hours after administration.
Compared with one-hour tracer uptake, four-hour uptake yielded higher lesional standardized uptake value (SUV) (mean, 33.2 vs. 23.3) and lower liver and bone background SUVs.
Cu-61 PSMA I&T PET/CT allowed for the detection of more lesions (65 versus 48 lesions) than F-18 piflufolastat PET/CT.
“A phase II trial of Cu-61 PSMA I&T has been designed to continue obtaining safety data for the radiotracer and to statistically determine noninferiority or superiority relative to another PSMA-targeting imaging agent, using pathology as the reference standard,” the researchers wrote.
They suggested that more broadly, the success of this first Cu-61-labeled PET tracer provides new opportunities for the construction of other, novel Cu-61 radiotracers for the treatment of multiple malignancies.
In an accompanying editorial, Partha Sinha, MD, of the University at Buffalo and the University of Kentucky, and Riham El Khouli, MD, PhD, of the University of Kentucky, noted that the longer half-life of the Cu-61 labeled tracer would offer advantages in the logistics of its manufacturing and distribution, as well as the possibility of delayed imaging, which can enhance sensitivity.
Moreover, the PSMA I&T molecule can be labeled with Cu-67 to form a complementary therapeutic agent (a theranostics pair), they added.
“Future research should be directed toward investigating the incremental clinical benefits of 61Cu-PSMA I&T PET/CT over existing PSMA-based imaging, especially regarding the utility of delayed imaging for detecting additional lesions,” Sinha and Khouli concluded.
The full study is available here.
Whether you are a professional looking for a new job or a representative of an organization who needs workforce solutions - we are here to help.